产品中心
联系我们
销售专用:
地址:北京市海淀区西小口路66号中关村东升科技园C-1楼三层

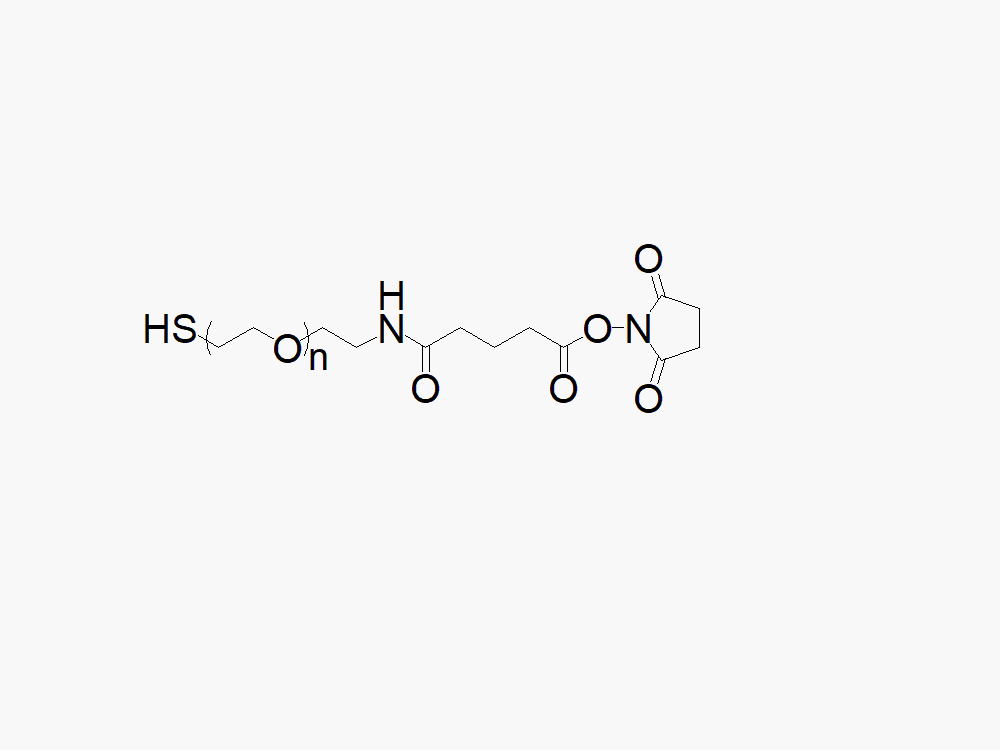
Amine PEG Acetic Acid, HCl Salt
产品代号:
HClNH2-PEG-CM
产品纯度:
≥ 95%
包装规格:
1g, 10g, 100g等(特殊包装需收取分装费用)
分子量:
1000 Da,2000 Da,3500 Da, 5000 Da, 7500 Da,10000 Da, 20000 Da,等
关键词:
所属分类:
科研客户小批量一键采购地址(小于5克)
- 产品描述
- 参考文献
-
键凯科技提供高品质胺基聚乙二醇羧酸盐酸盐,产品取代率大于等于95%。
键凯科技生产的异双功能胺PEG乙酸产品通常用作两种不同化学物质的交联剂或间隔物。此异功能PEG衍生物中的PEG部分可提供水溶性、生物相容性及柔性。此产品专门应用于抗体偶联药物(ADC’s)的开发。
键凯科技提供HClNH2-PEG-CM分子量1000 Da,2000 Da,3500 Da, 5000 Da, 7500 Da,10000 Da, 20000 Da,的产品1克和10克包装。
键凯科技提供分装服务,需要收取分装费用,如果您需要分装为其他规格请与我们联系。
键凯科技同时提供其他分子量的HClNH2-PEG-CM衍生物产品,如你需要请与我司sales@jenkem.com联系。
键凯科技提供大批量生产产品及GMP级别产品,如需报价请与我们联系。
-
References:
- Terracciano, M., et al., In Vivo Toxicity Assessment of Hybrid Diatomite Nanovectors Using Hydra vulgaris as a Model System, Advanced Biosystems, 2019.
- Lu, J., et al., Fabrication of thermo-and pH-sensitive cellulose nanofibrils-reinforced hydrogel with biomass nanoparticles, Carbohydrate Polymers, 2019.
- Terracciano, M., et al., Gold decorated porous biosilica nanodevices for advanced medicine, Nanotechnology, 2018, 29(23), p.235601.
- Kushwah, V., et al., Implication of linker length on cell cytotoxicity, pharmacokinetic and toxicity profile of gemcitabine-docetaxel combinatorial dual drug conjugate, International Journal of Pharmaceutics, 2018, V. 548 (1), P. 357-374.
- Alibolandi, M., et al., Tetrac-conjugated polymersomes for integrin-targeted delivery of camptothecin to colon adenocarcinoma in vitro and in vivo, International Journal of Pharmaceutics, 2017, V. 532 (1), p. 581-594.
- Xu, L., et al., Synthesis and Application of Injectable Bioorthogonal Dendrimer Hydrogels for Local Drug Delivery, ACS Biomaterials Science & Engineering, 2017.
- Zhao, L., et al., An intraocular drug delivery system using targeted nanocarriers attenuates retinal ganglion cell degeneration. Journal of Controlled Release, 2017.
- Sanna, V., et al., Targeted nanoparticles encapsulating (−)-epigallocatechin-3-gallate for prostate cancer prevention and therapy, Scientific Reports, 2017, 7:41573.
- Alpsoy, L., et al., Synthesis and Characterization of Carboxylated Luteolin (CL)-Functionalized SPION, Journal of Superconductivity and Novel Magnetism, 2017.
- Clawson, G.A., et al., A Cholecystokinin B Receptor-Specific DNA Aptamer for Targeting Pancreatic Ductal Adenocarcinoma, Nucleic acid therapeutics, 2017, 27(1):23-35.
- Ai, P., et al., The relative length of dual-target conjugated on iron oxide nanoparticles plays a role in brain glioma targeting, RSC Advances, 2017, 7(32):19954-9.
- Gao, D., et al., Targeted Ultrasound-Triggered Phase Transition Nanodroplets for Her2-Overexpressing Breast Cancer Diagnosis and Gene Transfection. Molecular Pharmaceutics, 2017, 14(4):984-98.
- Qi, Q., et al., Spatiotemporal delivery of nanoformulated liraglutide for cardiac regeneration after myocardial infarction, International journal of nanomedicine, 2017, 12:4835.
- Zhang, J., et al., Dual-targeting superparamagnetic iron oxide nanoprobes with high and low target density for brain glioma imaging, Journal of Colloid and Interface Science, 2016, V. 469, p. 86-92.
- Poojari, R., et al., Microtubule targeted therapeutics loaded polymeric assembled nanospheres for potentiation of antineoplastic activity, Faraday Discuss., 2016.
- Dasargyri, A., et al., Findings questioning the involvement of Sigma-1 receptor in the uptake of anisamide-decorated particles, Journal of Controlled Release, 2016, V. 224, p. 229-238.
- Alibolandi, M., et al., Folate receptor-targeted multimodal polymersomes for delivery of quantum dots and doxorubicin to breast adenocarcinoma: In vitro and in vivo evaluation, International Journal of Pharmaceutics, 2016, V. 500:1–2, p. 162-178.
- Bhargava-Shah, A., et al., Orlistat and antisense-miRNA-loaded PLGA-PEG nanoparticles for enhanced triple negative breast cancer therapy, Nanomedicine, 2016, 11: 3, P. 235-247.
- Spring, B.Q., et al., A photoactivable multi-inhibitor nanoliposome for tumour control and simultaneous inhibition of treatment escape pathways, Nature nanotechnology, 2016.
- Akal, Z., et al., Superparamagnetic iron oxide conjugated with folic acid and carboxylated quercetin for chemotherapy applications, Ceramics International, 2016, 42(7):9065-72.
- Brinkman, A.M., et al., Aminoflavone-loaded EGFR-targeted unimolecular micelle nanoparticles exhibit anti-cancer effects in triple negative breast cancer. Biomaterials, 2016, 101:20-31.
- Li, Q., et al., Development of reactive oxygen species (ROS)-responsive nanoplatform for targeted oral cancer therapy. Journal of Materials Chemistry B, 2016.
- Cao, L.B., et al., Highly Stable PEGylated Poly (lactic-co-glycolic acid)(PLGA) Nanoparticles for the Effective Delivery of Docetaxel in Prostate Cancers, Nanoscale Research Letters, 2016, 11(1):1.
- Wang, C., et al., Multi-functionalized graphene oxide complex as a plasmid delivery system for targeting hepatocellular carcinoma therapy, RSC Advances, 2016, 6(27):22461-8.
- Thapa, R.K., et al., Liquid crystalline nanoparticles encapsulating cisplatin and docetaxel combination for targeted therapy of breast cancer, Biomaterials Science, 2016.
- Devulapally, R., et al., Polymer Nanoparticles Mediated Codelivery of AntimiR-10b and AntimiR-21 for Achieving Triple Negative Breast Cancer Therapy, ACS Nano, 2015, 9(3): 2290-2302.
- Liu, S., et al., Meter-long multiblock copolymer microfibers via interfacial bioorthogonal polymerization, Adv. Mater., 2015.
- Sun, N., et al., A Cellular Compatible Chitosan Nanoparticle Surface for Isolation and In Situ Culture of Rare Number CTCs, Small, 2015, 11: 5444–5451.
- Poojari, R., et al., A Chimeric Cetuximab-Functionalized Corona as a Potent Delivery System for Microtubule-Destabilizing Nanocomplexes to Hepatocellular Carcinoma Cells: A Focus on EGFR and Tubulin Intracellular Dynamics, Molecular Pharmaceutics, 2015, 12 (11), 3908-3923.
- Bielski, E. R., et al., Effect of the Conjugation Density of Triphenylphosphonium Cation on the Mitochondrial Targeting of Poly(amidoamine) Dendrimers, Molecular Pharmaceutics, 2015, 12 (8), 3043-3053.
- Devulapally, R., et al., Formulation of Anti-miR-21 and 4-Hydroxytamoxifen Co-loaded Biodegradable Polymer Nanoparticles and Their Antiproliferative Effect on Breast Cancer Cells, Molecular Pharmaceutics, 2015.
- Kang, T., et al., Enhancing Glioblastoma-Specific Penetration by Functionalization of Nanoparticles with an Iron-Mimic Peptide Targeting Transferrin/Transferrin Receptor Complex, Molecular Pharmaceutics, 2015, 12 (8), 2947-2961.
- Alibolandi, M., et al., Epithelial cell adhesion molecule aptamer conjugated PEG–PLGA nanopolymersomes for targeted delivery of doxorubicin to human breast adenocarcinoma cell line in vitro, International Journal of Pharmaceutics, 2015, 479:1, P. 241-251.
- Vandana, M., et al., Synergistic activity of combination therapy with PEGylated pemetrexed and gemcitabine for an effective cancer treatment, European Journal of Pharmaceutics and Biopharmaceutics, 2015, 94, P. 83-93.
- Lo, Y.L., et al., Folic Acid Linked Chondroitin Sulfate-Polyethyleneimine Copolymer Based Gene Delivery System, Journal of Biomedical Nanotechnology, 2015, 11:8, p. 1385-1400(16).
- Alibolandi, M., et al., In vitro and in vivo evaluation of therapy targeting epithelial-cell adhesion-molecule aptamers for non-small cell lung cancer, Journal of Controlled Release, 2015, 209, P. 88-100.
- Terracciano, M., et al., Surface bioengineering of diatomite based nanovectors for efficient intracellular uptake and drug delivery, Nanoscale, 2015, 7, 20063-20074.
- Zhang, X.-Q., et al., Nanoparticles Containing a Liver X Receptor Agonist Inhibit Inflammation and Atherosclerosis. Advanced Healthcare Materials, 2015, 4: 228–236.
- Yang, L., et al., Photothermal Targetting therapy of A20 Mouse Lymphoma model using Anti CD-138 Antibody conjugated Gold Nanospheres, J Hematol Thrombo Dis, 2014, 2:3.
- Kang, T., et al., iNGR-modified PEG-PLGA nanoparticles that recognize tumor vasculature and penetrate gliomas. Biomaterials, 2014, 35(14): p. 4319-4332.
- Baker, D.W., et al., Development of optical probes for in vivo imaging of polarized macrophages during foreign body reactions. Acta Biomaterialia, 2014, 10(7): p. 2945-2955.
- Jain, S., et al., Combinatorial bio-conjugation of gemcitabine and curcumin enables dual drug delivery with synergistic anticancer efficacy and reduced toxicity, RSC Adv., 2014, 4, 29193-29201.
- Gu, W., et al., Chlorotoxin-conjugated, PEGylated Gd2O3 nanoparticles as a glioma-specific magnetic resonance imaging contrast agent, RSC Adv., 2014, 4, 50254-50260.
- Liu, Y.-S., Folate-mediated and doxorubicin-conjugated poly(ε-caprolactone)-g-chondroitin sulfate copolymers for enhanced intracellular drug delivery, RSC Adv., 2014, 4, 59548-59557.
- Zhou, W., et al., Aptamer-nanoparticle bioconjugates enhance intracellular delivery of vinorelbine to breast cancer cells, Journal of Drug Targeting, 2014, 22:1.
- Schuster, B.S.,et al., Overcoming the cystic fibrosis sputum barrier to leading adeno-associated virus gene therapy vectors, Mol Ther., 2014, 22(8):1484-93.
- Xiao, B., et al., Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-α RNA interference for IBD therapy, Biomaterials, 2013, 34(30), p:7471-7482.
- Sanna, V., et al, Resveratrol-Loaded Nanoparticles Based on Poly(epsilon-caprolactone) and Poly(d,l-lactic-co-glycolic acid)–Poly(ethylene glycol) Blend for Prostate Cancer Treatment, Mol. Pharmaceutics, 2013, 10(10), p:3871–3881.
- Han, H., et al., Targeted Nanoparticles Assembled via Complexation of Boronic-Acid-Containing Targeting Moieties to Diol-Containing Polymers, Bioconjugate Chem., 2013, 24(4), p: 669–677.
- Jain, R., et al., Antitumor activity of a monoclonal antibody targeting major histocompatibility complex class I–Her2 peptide complexes, Journal of the National Cancer Institute, 2013, 105.3, 202-218.
- Baker, D.W., The Pivotal Role Of Fibrocytes On Foreign Body Reactions, UTA, 2013.
- Vandana, M., et al., Reduced Folate Carrier Independent Internalization of PEGylated Pemetrexed: A Potential Nanomedicinal Approach for Breast Cancer Therapy, Mol. Pharmaceutics, 2012, 9(10), p: 2828–2843.
- Sanna, V., et al., Targeted Biocompatible Nanoparticles for the Delivery of (−)-Epigallocatechin 3-Gallate to Prostate Cancer Cells, Journal of Medicinal Chemistry, 2011, 54 (5), 1321-1332.
- Guo, J., et al., Aptamer-functionalized PEG–PLGA nanoparticles for enhanced anti-glioma drug delivery, Biomaterials, 2011, 32:31, P. 8010-8020.
- Sanna, V., et al, Development of Polymeric Microbubbles Targeted to Prostate-Specific Membrane Antigen as Prototype of Novel Ultrasound Contrast Agents, Mol. Pharmaceutics, 2011, 8 (3), pp 748–757.
- Peng Zou, et al., Superparamagnetic Iron Oxide Nanotheranostics for Targeted Cancer Cell Imaging and pH-Dependent Intracellular Drug Release, Mol. Pharmaceutics, 2010, 7(6) p: 1974–1984.
- Biswal, B.K., et al., Development of a targeted siRNA delivery system using FOL-PEG-PEI conjugate, Molecular Biology Reports, 2010, 37:6, p. 2919-2926.
- Lu, W., et al., Targeted Photothermal Ablation of Murine Melanomas with Melanocyte-Stimulating Hormone Analog–Conjugated Hollow Gold Nanospheres, Clinical Cancer Research, 2009, 15; 876.
- Aumsuwan, N., et al., Attachment of Ampicillin to Expanded Poly(tetrafluoroethylene): Surface Reactions Leading to Inhibition of Microbial Growth, Biomacromolecules, 2008, 9(7), p: 1712–1718.
- Li, Z., et al., Nanoparticle depots for controlled and sustained gene delivery, Journal of Controlled Release, 2020.
- Lumen, D., et al., Investigation of silicon nanoparticles produced by centrifuge chemical vapor deposition for applications in therapy and diagnostics, European Journal of Pharmaceutics and Biopharmaceutics, 2021, V. 158, P. 254-265.
-
Poojari, R. et al., Distinct stratification of normal liver, hepatocellular carcinoma (HCC), and anticancer nanomedicine-treated- tumor tissues by Raman fingerprinting for HCC therapeutic monitoring, Nanomedicine: Nanotechnology, Biology and Medicine, 2021, V. 33.
-
Sheng, Q, et al., Comprehensively enhanced delivery cascade by transformable beaded nanofibrils for pancreatic cancer therapy. Nanoscale. 2021.
-
Deng, M, et al., pH-Triggered Copper-Free Click Reaction-Mediated Micelle Aggregation for Enhanced Tumor Retention and Elevated Immuno–Chemotherapy against Melanoma. ACS Applied Materials & Interfaces. 2021, 13(15):18033-46.
-
Guo, L.Y., et al., Skin-safe nanophotosensitizers with highly-controlled synthesized polydopamine shell for synergetic chemo-photodynamic therapy, Journal of Colloid and Interface Science, 2022, 616, pp.81-92.
-
Damrongrak, K., et al., Delivery of acetogenin-enriched Annona muricata Linn leaf extract by folic acid-conjugated and triphenylphosphonium-conjugated poly (glycerol adipate) nanoparticles to enhance toxicity against ovarian cancer cells, International Journal of Pharmaceutics, 2022, 121636.
-
Poellmann, M. J., et al., Circulating tumor cell abundance in head and neck squamous cell carcinoma decreases with successful chemoradiation and cetuximab treatment, Cancer Letters, 2023, V. 562.
-
Shirasu, T., et al., Neointima abating and endothelium preserving — An adventitia-localized nanoformulation to inhibit the epigenetic writer DOT1L, Biomaterials, 301, 2023.
产品询价